DCGI approves DRDO-developed anti-COVID Drug for emergency use
DCGI approves DRDO-developed anti-COVID Drug
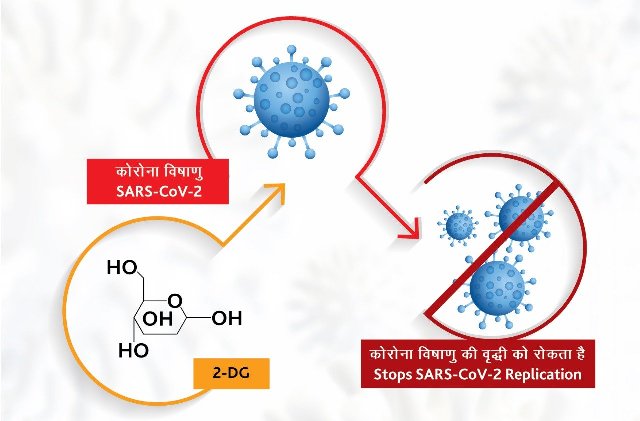
TNI Bureau: The Drugs Controller General of India (DCGI) on Saturday approved anti-COVID drug 2-deoxy-D-glucose (2-DG) for emergency use.
The drug developed by the Institute of Nuclear Medicine and Allied Sciences (INMAS), a leading laboratory of Defence Research and Development Organisation (DRDO) in collaboration with Dr Reddy’s Laboratories, Hyderabad will help in faster recovery of hospitalised Covid patients and reduce supplemental oxygen dependence.
Support Independent Journalism? Keep us live.
The third phase of the clinical trial results have shown that the drug may save precious lives due to the mechanism of operation of the drug in infected cells, an official release said.
The anti-COVID drug 2-deoxy-D-glucose (2-DG) comes in powder form in sachet, which is taken orally by dissolving it in water.
Being a generic molecule & analogue of glucose, it can be easily produced & made available in plenty, informed DRDO.

Comments are closed.